Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially curative treatment modality for patients with myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML). Non-myeloablative (NMA) conditioning regimens are used to decrease toxicity, making HSCT more tolerable and available to older patients or patients with co-morbidities. It remains unclear as to which NMA regimen leads to superior outcomes.
Methods: We retrospectively compared outcomes of 3 NMA regimens in 98 patients with AML or MDS undergoing allogeneic HSCT from January 2017 to December 2021. The 3 regimens were: Flu/Mel 100: fludarabine 40 mg/m2 days -5 to -2, melphalan 100 mg/m2 day -2; Flu/Bu2: fludarabine 30 mg/m2 days -6 to -2, busulfan 130 mg/m2 days -3 to -2; Flu/TBI: fludarabine 30 mg/m2 days -4 to -2, total body irradiation 200-300 cGY on day-1. Patients conditioned with Flu/Mel 100 or Flu/Bu2 also received rabbit anti-thymocyte globulin 4-6 mg/kg. Graft versus host disease (GVHD) prophylaxis consisted of tacrolimus and low dose methotrexate for patients receiving Flu/Mel 100 or Flu/Bu2, and sirolimus, cyclosporine and mycophenolate mofetil for patients conditioned with Flu/TBI.
Continuous variables were compared between conditioning regimens using Pearson's Chi-squared test or Fisher's exact test. PFS and OS survival probability and survival times were estimated with Kaplan-Meyer(K-M) procedure. To compare survival distributions between conditioning regimens, a log-rank test was used. We report 24-month survival probabilities in months with corresponding 95% confidence intervals.
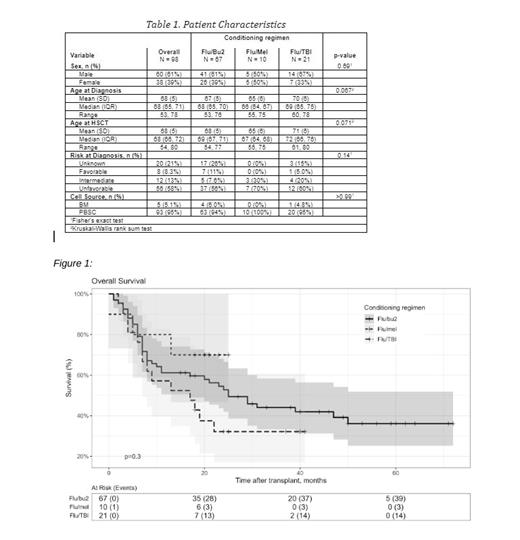
Results: Patient demographics were balanced between the 3 groups including age (range 54-80), gender, disease risk and cell source (Table 1). 24-month progression free survival (PFS) was not different between the groups: Flu/Mel 100 was 70% (47%-100%), Flu/Bu2 was 49% (39%-63%), and Flu/TBI was 33% (18%-61%) (p=0.3). For Flu/Mel 100 the 24-month overall survival (OS) was 70% (47%-100%), Flu/Bu2 was 53% (42%-66%), Flu/TBI was 32% (17%-61%) which was not statistically different ( p=0.3) (Figure 1). There was no difference between the 3 groups for non-relapse mortality (NRM) ( p=0.46). The incidence of all grade acute GVHD was 70% for Flu/Mel 100, 66% for Flu/Bu2 and 76% for Flu/TBI ( p=0.70). There was no difference in the incidence of chronic GVHD between the groups; Flu/Mel 100 50%, Flu/Bu2 55%, Flu/TBI 57% (p>0.99).
Conclusions: All three NMA regimens were found to be statistically equivalent. NRM, PFS, OS and the incidence of acute and chronic GVHD was not statistically different between the groups. A limitation of the study is the relatively small number of patients in the Flu/Mel 100 group. Further study with a larger sample size is needed to evaluate for small differences between the regimens.
Disclosures
McKiernan:Sanofi: Speakers Bureau. Suh:Kite Pharma: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal